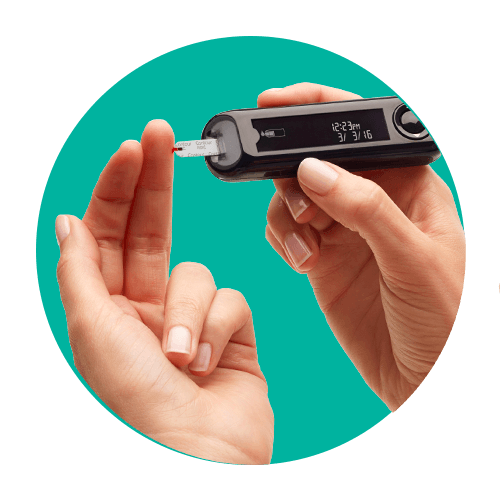
Insert the test strip with the gray square end facing the meter (this will automatically turn on the meter).
CONTOUR®PLUS ONE
The CONTOUR®PLUS ONE blood glucose meter offers seamless connection to the CONTOUR®DIABETES app to capture all your blood glucose readings.
Product Features
- Simple & Intuitive
- smartLIGHT® for easier understanding of blood glucose levels1
- Highly accurate2
- Save strips with Second-Chance® Sampling technology
- Connects to the free CONTOUR®DIABETES app**
Why choose CONTOUR®PLUS ONE?
Easy to use. Fast results
Simple to set up and easy-to-use. Get your results in 5 seconds. For patients who want a simple-to-use meter which seamlessly connects to their smartphone to help them manage their diabetes, smarter.

Discover smartLIGHT® for easier understanding of blood glucose levels1
The unique smartLIGHT® feature makes it quicker and easier to interpret blood glucose readings using coloured lights that clearly identify if the reading is above, within or below your target range.†

Highly accurate2
The CONTOUR®PLUS ONE system exceeded the minimum accuracy requirements of the ISO 15197:2013 (EN ISO 15197:2015) standards.* 2,3

Can save strips with Second-Chance® Sampling technology
Second-Chance® sampling allows you to reapply more blood to the same test strip when the first sample is insufficient1, to help you waste fewer strips.4

Connects to the free CONTOUR®DIABETES app
The free CONTOUR®DIABETES app is available to support diabetes self-management, adding insight and meaning to the results
Easy to use: Syncs automatically with the meter to upload all blood glucose readings to an electronic diary
Easy to understand: My Patterns helps identify trends in blood glucose results and displays notifications of potential causes
Easy to share: The blood sugar diary report can be sent prior to, or shared during, an appointment with a healthcare professional
The CONTOUR®DIABETES app applies appropriate safeguards to ensure your personal data is processed securely and in compliance with applicable laws.
Check to see if your smartphone’s operating system is compatible
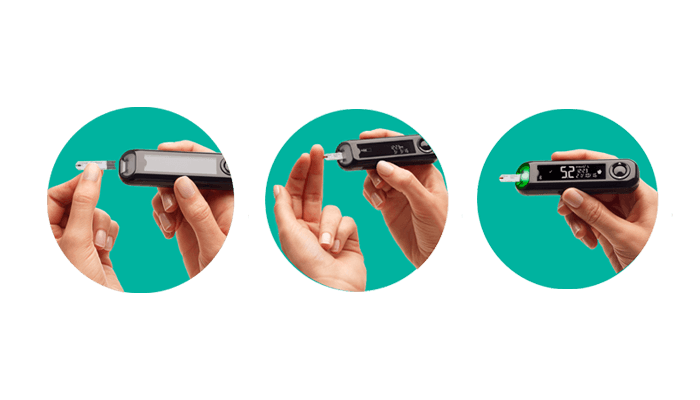
How to use*
You are only 3 steps away from a remarkably accurate1 result
Works With
Please consult your healthcare professional for interpretation of result and diagnosis.
*95% of the measured glucose values needed to fall within either ±0.83 mmol/L (±15 mg/dL) of the average measured values of the reference measurement procedure at glucose concentrations <5.55 mmol/L (<100 mg/dL) or within ±15% at glucose concentrations ≥5.55 mmol/L (≥100 mg/dL). 99% of individual glucose measured values need to fall within zones A and B of the Consensus Error Grid (CEG) for type 1 diabetes.3
**On a compatible Android or iOS device. For a list of compatible operating systems, please visit compatibility.contourone.com
1. CONTOUR®PLUS ONE BGMS User Guide Rev 09/17.
2. Bailey TS. J Diabetes Sci Technol 2017 Jul;11(4):736-743.
3. International Organization for Standardization. In vitro diagnostic test systems - requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO 15197). International Organization for Standardization, Geneva, Switzerland, 2013.
4. Richardson JM et al. Clinical Relevance of Reapplication of Blood Samples During Blood Glucose Testing. Poster presented at the 20th Annual Diabetes Technology Meeting (DTM); November 12-14, 2020.
Registered under Act 737 and MDA registration no IVDC 33901325718
MD Advertisement approval number: MDAMD 0181/2023